Answer:
d) BaF2
Step-by-step explanation:
The compound which is more soluble in an acidic solution than in a neutral solution is shown below:-
First we will compare acidic with Neutral
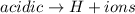
So,
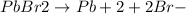
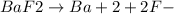
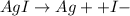
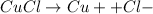
Now, when we add H+ ions, so it will be
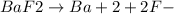

Then it will reduces F-, as BaF2 begin to form more aqueous ions, so, it will rises the solubility