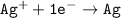Answer:
Step-by-step explanation:
We are to write a chemical equation for the reaction that occurs in the following cell: Cu|Cu2+(aq)||Ag+(aq)|Ag.
And to determine which one of the two metals, (copper and silver) is the anode and which is the cathode?
The chemical equation for the reaction is as follows:
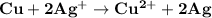
The atoms of the Copper electrodes undergo oxidation and loses two electrons each to form copper II ions
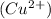 which go into the solution. The copper then becomes negatively charged and functions as the negative electrode i.e the anode
which go into the solution. The copper then becomes negatively charged and functions as the negative electrode i.e the anode
The oxidation half reaction is:
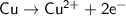
The silver ions
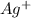 becomes reduced by gaining two electrons each from the metallic copper which was deposited into the silver electrode. The silver electrode thus becomes positively charged and functions as the positive electrode. i.e the cathode.
becomes reduced by gaining two electrons each from the metallic copper which was deposited into the silver electrode. The silver electrode thus becomes positively charged and functions as the positive electrode. i.e the cathode.
The reduction half reaction is: