Answer:
Normality N = 0.2 N
Step-by-step explanation:
Normality is the number of gram of equivalent of solute divided of volume of solution, where the number of gram of equivalent of solute is weight of the solute divided by the equivalent weight.
Normality is represented by N.
Mathematically, we have :
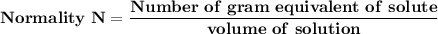
Given that:
number of gram of equivalent of solute = 90 milliequivalents 90 × 10⁻³ equivalent
volume of solution (HCl) = 450 mL 450 × 10⁻³ L
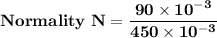
Normality N = 0.2 N