Answer:
the expected absorbance of the solution = 0.17
Step-by-step explanation:
From the information given:
Using Beer's Lambert Law, we have
A = ∈CL
where;
A = Absorbance
∈ = extinction coefficient
C = concentration
L = cell length
Since Absorbance is associated with concentration.
Assuming the measurement were carried out in the same solution; Then ∈ and L will be constant and A ∝ C ----- (1)
Let consider the concentration to be C (mol/L)
5.0 mL of a Sports Drink = 5.0 mL × C (mol)/1000 mL
= 5C/1000 mL
was diluted with water to 10.0 mL
So, when diluted with water to 10.0 mL; we have:
The new concentration to be :
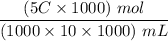
Since :1000mL = 1 L
The new concentration =
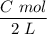
As stated that the initial absorbance reading
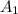 = 0.34
= 0.34
The expected absorbance reading will be
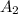 = ???
= ???
From (1)
A ∝ C
∴
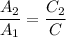
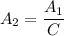
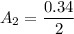
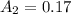
Thus ; the expected absorbance of the solution = 0.17