Answer:
The correct answer is "C. Qc > Kc, the reaction proceeds from right to left to reach equilibrium"
Step-by-step explanation:
The reaction quotient Qc is a measure of the relative amount of products and reactants present in a reaction at a given time.
Being:
aA + bB ⇔ cC + dD
where a, b, c and d are the stoichiometric coefficients of the balanced equation, the coefficient Q is calculated as:
![Qc=([C]^(c)*[D]^(d) )/([A]^(a) *[B]^(b) )](https://img.qammunity.org/2021/formulas/chemistry/high-school/l8lu6gpexmq1u5m8ons98dhvpnfham08h4.png)
If Qc <Kc there is less concentration of products than in equilibrium, with which the reaction will evolve to the right to increase the concentration of products.
If Qc> Kc, it is possible to affirm that the reaction will evolve to the left since in this case the direct reaction predominates and there will be more product present than what is obtained in equilibrium. Therefore, this product is used to promote the reverse reaction and achieve equilibrium. Then the system will evolve to the left to increase the concentration of reagents.
If Qc = Kc, it means that the reaction is in equilibrium.
In the case of the reaction:
2 SO₂ (g) + O₂ (g) ⇔ 2 SO₃(g)
the value of the constant Qc is calculated as:
![Qc=([SO_(3) ]^(2) )/([SO_(2) ]^(2) *[O_(2) ])](https://img.qammunity.org/2021/formulas/chemistry/college/n09x66k3xuu2t3h2orgyuqp2gjv2dsan8i.png)
Being:
- [SO₂] = 0.010 M
- [SO₃] = 10 M
- [O₂] = 0.010 M
and replacing:
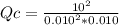
you get:
Qc=100,000,000=1*10⁸
Being Kc=4.3*10⁶, then Qc>Kc and the reaction proceeds from right to left to reach equilibrium.
So the correct answer is "C. Qc > Kc, the reaction proceeds from right to left to reach equilibrium"