Answer:
b. Q
Step-by-step explanation:
Equilibrium constant K is a measure of the ratio of the equilibrium concentration of the products of a reaction to the equilibrium concentration of the reactants with each concentration raised to the power corresponding to the coefficient in the balanced equation of the reaction.
On the other hand , the
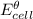 is the standard electrode potential of the right-hand electrode minus the standard electrode potential of the left hand electrode, Thus,
is the standard electrode potential of the right-hand electrode minus the standard electrode potential of the left hand electrode, Thus,
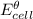 will be zero if concentration cell in the two electrodes appears to be the same.
will be zero if concentration cell in the two electrodes appears to be the same.
The Nernst equation correlates the cell E.M.F to a standard value E and the activities of the species that takes places in the cell reaction. Thus in a concentration cell, the reaction is driven by Q