Answer:
0.16 moles of Carbon
Step-by-step explanation:
The balanced reaction equation:
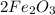 +
+
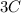 →
→
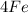 +
+
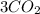 ↑
↑
The mole ratio of Carbon to Iron is 3 : 4 (since Fe2O3 is in excess)
i.e 3 moles of C produces 4 moles of Fe.
If 1 mole of Fe - 55.8g of Fe
? moles - 11.6g of Fe
=
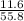 = 0.208 moles
= 0.208 moles
But 3 moles of C - 4 moles of Fe
? moles of C - 0.208 moles of Fe
=
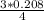 = 0.16 moles of carbon.
= 0.16 moles of carbon.
I hope this explanation was clear and useful.