Answer:
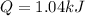
Step-by-step explanation:
Hello,
In this case, for latent heat (phase change) we need to consider the enthalpy associated with the involved process, here, melting or fusion; thus, the enthalpy of fusion of copper is 13.2 kJ/mol, therefore, the heat is computed as:
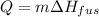
Nevertheless, since the given enthalpy is per mole of copper, we need to use its atomic mass to perform the correct calculation as follows:
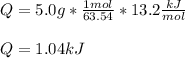
Which is positive as it needs to be supplied to the system.
Best regards.