Answer:
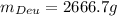
Step-by-step explanation:
Hello,
In this case, we can apply the following mole-mass-density relationship in order to compute the required grams of deuterium, considering that it is the 0.0150% (molar basis) of natural hydrogen (H₂):
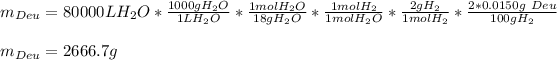
Best regards.