Answer:
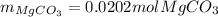
Step-by-step explanation:
Hello,
In this case, for this purpose we first have to write the undergoing chemical reaction:
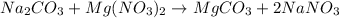
Thus, since the mole ratio between the reactants is 1:1, we next identify the limiting reactant by computing the available moles of sodium carbonate and those moles of the same reactant consumed by the magnesium nitrate considering the given solutions:
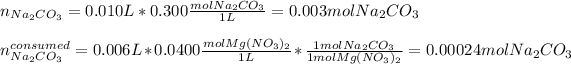
In such a way, since less moles are consumed, we can say that the sodium carbonate is excess whereas the magnesium nitrate is the limiting one, therefore, the yielded mass of magnesium carbonate turns out:
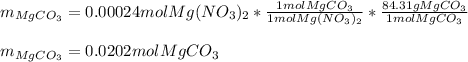
Regards.