Answer:
6.624 x 10^-21 J
Step-by-step explanation:
The temperature of the ideal gas = 320 K
The average translational energy of an ideal gas is gotten as
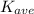 =
=
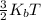
where
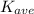 is the average translational energy of the molecules
is the average translational energy of the molecules
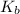 = Boltzmann constant = 1.38 × 10^-23 m^2 kg s^-2 K^-1
= Boltzmann constant = 1.38 × 10^-23 m^2 kg s^-2 K^-1
T is the temperature of the gas = 320 K
substituting value, we have
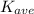 =
=
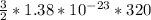 = 6.624 x 10^-21 J
= 6.624 x 10^-21 J