Answer:
2.15
Step-by-step explanation:
For this question, we have to remember the pH formula:
![pH~=~-Log[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/s1dphmwi2twq1qzacfhbacuhqjpdypntka.png)
By definition, the pH value is calculated when we do the -Log of the concentration of the hydronium ions (
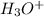 ). So, the next step is the calculation of the concentration of the hydronium ions. For this, we have to use the molarity formula:
). So, the next step is the calculation of the concentration of the hydronium ions. For this, we have to use the molarity formula:
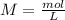
We already know the number of moles (0.0231 moles) and the volume (3.33 L). So, we can plug the values into the molarity formula:
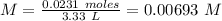
With this value, now we can calculate the pH value:
![pH~=~-Log[0.00693~M]~=~2.15](https://img.qammunity.org/2021/formulas/chemistry/college/wxyzbkjpfsycg8s7wla9z3bds2ad2dw9su.png)
The pH would be 2.15
I hope it helps!