Answer:
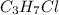 = Two structures
= Two structures
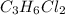 = Four structures
= Four structures
Step-by-step explanation:
We must remember that in an isomer we have the same molecular formula but different structures. Thus, for the molecule
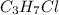 we can draw a linear structure of 3 carbons and change the position of the chlorine atom, obtaining two different structures.
we can draw a linear structure of 3 carbons and change the position of the chlorine atom, obtaining two different structures.
For the molecule
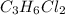 , we can use similar logic. Place a chain of 3 carbons and change the position of the chlorine atoms in such a way that for this formula we will have 4 different structures.
, we can use similar logic. Place a chain of 3 carbons and change the position of the chlorine atoms in such a way that for this formula we will have 4 different structures.
See figures 1 and 2 for further explanations.