Answer:
See explanation
Step-by-step explanation:
In this case, we can start with the half-reactions. If the total reaction is:
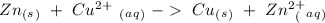
If we split the reaction we will have:
Half-reaction 1:
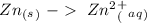
Half-reaction 2:
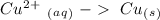
Now we can add the electrons, keeping in mind that we have to obtain zero charge in both sides of each half-reaction:
Half-reaction 1:
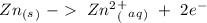
Half-reaction 2:
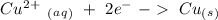
With this in mind, we can solve the questions:
Which one loses electrons?
In half-reaction number 1 we have the electrons in the products side, therefore this half-reaction is the one that loses electrons.
Which one gains electrons
In half-reaction number 2 we have the electrons in the reagent side, therefore this half-reaction is the one that gains electrons.
Which one is oxidized?
If half-reaction number 1 loses electrons will be the oxidation reaction.
Which one is reduced?
If half-reaction number 2 gains electrons will be the reduction reaction.
Which species functions as the oxidizing agent in the following reduction-oxidation reaction?
If half-reaction number 2 is the reduction will be a oxidizing agent.
Which one the reducing agent?
If half-reaction number 1 is the oxidation will be a reducing agent.
I hope it helps!