Answer:
-
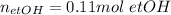
-
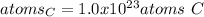
Step-by-step explanation:
Hello,
In this case, since ethanol has a molar mass of 46 g/mol, the moles in 5.0 g are:
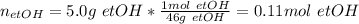
Moreover, since the formula of glucose is C₆H₁₂O₆, its molar mass is 180 g/mol and six moles of carbon are in one mole of glucose (based on carbon's subscript), the atoms are computed by using the 6:1 mole ratio and the Avogadro's number as shown below:
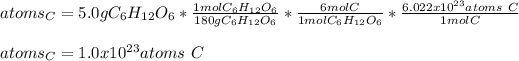
Regards.