Answer:
Vapor pressure of the solution is 22.9 torr at 25°C
Step-by-step explanation:
When a non volatile solute is added to a pure solvent, the vapour pressure of the mixture decrease regard to the pure solvent following the equation (Raoult's law):
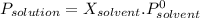
Where vapour pressure of the pure solvent (Water) is 23.8torr at 25°C.
Mole fraction of water is the ratio between moles of water and total moles in solution. The solution has a mole fraction of water of:
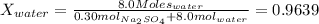
Replacing in Raoult's law:
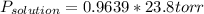
Vapor pressure of the solution is 22.9 torr at 25°C