Answer:
The molar mass of the unknown is approximate 145g/mol
Step-by-step explanation:
We can relate the speed of effusion of gases with the molar mass of the 2 compounds using Graham´s law:
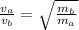
Where V is speed of the gas and m molar mass of a and b gases.
Molar mass of helium is 4g/mol. As the distance of the gases is the same, we can replace the speed of effusion with the time the gas takes to travel the distance. The equation will be:
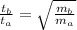
Replacing:
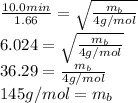
The molar mass of the unknown is approximate 145g/mol