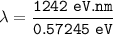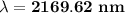Answer:
the wavelength of radiation emitted is
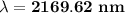
Step-by-step explanation:
The energy of the Bohr's hydrogen atom can be expressed with the formula:
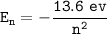
For n = 7:
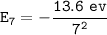
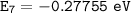
For n = 4
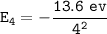
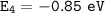
The electron goes from the n = 7 to the n = 4, then :
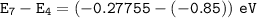
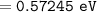
Wavelength of the radiation emitted:
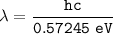
where;
hc = 1242 eV.nm