Answer:
The mass of nitrogen molecule
 = 45.65 g
= 45.65 g
Step-by-step explanation:
The equation for the redox reaction can be represented as follows:
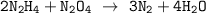
We know that:
numbers of moles = mass/molar mass
For
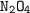 :
:
number of moles = 50g/92 g/mol
number of moles = 0.5435 mol
For
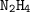 :
:
number of moles = 45 g/ 32 g/mol
number of moles = 1.40625 mol
From the above equation;
number of moles of
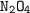 needed = 1/2 moles of
needed = 1/2 moles of
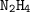 = 1/2 × 1.40625 mol
= 1/2 × 1.40625 mol
= 0.703125 mol
The amount of
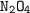 present = 0.5435 moles which is less than the needed. As such
present = 0.5435 moles which is less than the needed. As such
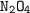 is the limiting reagent
is the limiting reagent
The number of moles of nitrogen molecule
 produced = 3 × (
produced = 3 × (
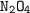 )
)
= 3 × 0.5435
= 1.6305 mol
The mass of nitrogen molecule
 = number of moles of
= number of moles of
 × molar mass of
× molar mass of

The mass of nitrogen molecule
 = 1.6305 mol × 28 g/mol
= 1.6305 mol × 28 g/mol
The mass of nitrogen molecule
 = 45.654 g
= 45.654 g
The mass of nitrogen molecule
 = 45.65 g
= 45.65 g