Answer:
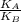 = 1
= 1
Step-by-step explanation: Average molecular kinetic energy for monatomic idela gases is given by:
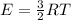
where
R is gas constant of ideal gas
T is temperature
Which means kinetic energy depnends only on temperature.
Since gas A and gas B are at the same temperature, kinetic energy will be the same. Therefore:
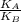 = 1
= 1
Ratio of this average molecular kinetic energy is 1.