Answer:
0.0630
Step-by-step explanation:
The molar mass of urea = 60 g/mol
we all know that:
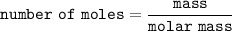
Then; the number of moles of urea
=
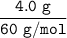
= 0.0667 mol
Similarly; the number of moles of methanol
=
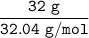
= 0.9988 mol
The total number of moles = (0.0667 + 0.9988) mol
= 1.0655 mol
Finally,the mole fraction of urea
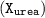 =
=
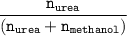
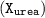 =
=
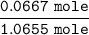
= 0.0630