Answer:
The maximum wavelength of incident light that can produce photoelectrons from silver is 423.5 nm.
Step-by-step explanation:
Given;
work function of silver, Φ = 2.93 eV = 2.93 x 1.602 x 10⁻¹⁹ J = 4.6939 x 10⁻¹⁹ J
Apply Einstein Photo electric effect;
E = K.E + Ф
Where;
E is the energy of the incident light
K.E is the kinetic of electron
Ф is the work function of silver surface
For the incident light to have maximum wavelength, the kinetic energy of the electron will be zero.
E = Ф
hf = Ф
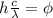
where;
c is speed of light = 3 x 10⁸ m/s
h is Planck's constant, = 6.626 x 10⁻³⁴ J/s
λ is the wavelength of the incident light
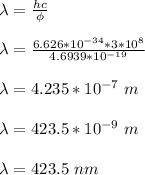
Therefore, the maximum wavelength of incident light that can produce photoelectrons from silver is 423.5 nm.