Answer:
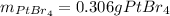
Step-by-step explanation:
Hello,
In this case, since the solubility product of platinum (IV) bromide is 8.21x10⁻⁹, and the dissociation is:
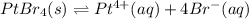
The equilibrium expression is:
![Ksp=[Pt^(4+)][Br^-]^4](https://img.qammunity.org/2021/formulas/chemistry/college/q3nn2lyzmrnh8ssanhgytvyc0e9i8bx892.png)
Thus, since the salt is added to a solution initially containing 1.00 grams of potassium bromide, there is an initial concentration of bromide ions:
![[Br^-]_0=(1.00gKBr*(1molKBr)/(119gKBr)*(1molBr^-)/(1molKBr) )/(0.250L)=0.0336M](https://img.qammunity.org/2021/formulas/chemistry/college/z2h22n9bwpvr46cs2j9x6u6odb39tk1d7d.png)
Hence, in terms of the molar solubility
 , we can write:
, we can write:
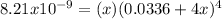
In such a way, solving for
 , we obtain:
, we obtain:
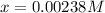
Which is the molar solubility of platinum (IV) bromide. Then, since its molar mass is 514.7 g/mol, we can compute the grams that get dissolved in the 250.0-mL solution:
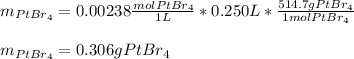
Best regards.