Answer:
2-methyl-2-pentyl-1,3-dioxolane
Step-by-step explanation:
In this case, we have two reactions:
First reaction:
1-heptyne + mercuric acetate -------> Compound A
Second reaction:
Compound A + HOCH2CH2OH -------> Compound C
First reaction
In the first reaction, we have as a main functional group a triple bond. We have to remember that mercuric acetate in sulfuric acid will produce a ketone. The carbonyl group (C=O) would be placed in the most substituted carbon of the triplet bond (in this case, carbon 2). With this in mind, we will have as a product: heptan-2-one. (See figure 1).
Second reaction
In this reaction, we have as reagents:
-) Heptan-2-one
-) Ethylene-glycol
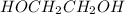
-) Sulfuric acid

When we put ethylene-glycol with a ketone or an aldehyde we will form a cyclic acetal. In this case, this structure would be formed on carbon 2 forming 2-methyl-2-pentyl-1,3-dioxolane. (See figure 2).
I hope it helps!