Answer:
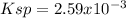
Step-by-step explanation:
Hello,
In this case, given the 0.0990 moles of the salt are soluble in 1.00 L of water only, we can infer that the molar solubility is 0.099 M. Next, since the dissociation of the salt is:
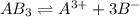
The concentrations of the A and B ions in the solution are:
![[A]=0.099 (molAB_3)/(L)*(1molA)/(1molAB_3) =0.0099M](https://img.qammunity.org/2021/formulas/chemistry/college/im5ow6ch7172mrs4g53ujjsmoraismxtqr.png)
![[B]=0.099 (molAB_3)/(L)*(3molB)/(1molAB_3) =0.000.297M](https://img.qammunity.org/2021/formulas/chemistry/college/mn8ve4lmha6r8w2eknew6o51a53o431yru.png)
Then, as the solubility product is defined as:
![Ksp=[A][B]^3](https://img.qammunity.org/2021/formulas/chemistry/college/k7wfqy41sk4i1dn74ort8ni9jxat75pe5j.png)
Due to the given dissociation, it turns out:
![Ksp=[0.099M][0.297M]^3\\\\Ksp=2.59x10^(-3)](https://img.qammunity.org/2021/formulas/chemistry/college/kygaxp78pf02tjmretlpt1gjqmlvow9ssd.png)
Regards.