Answer:
35 hrs
Step-by-step explanation:
half life of the substance
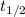 = 8.1 hr
= 8.1 hr
initial amount
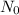 = 75 g
= 75 g
The final amount
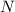 = 3.9 g
= 3.9 g
The time elapsed
 = ?
= ?
we use the relationship
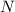 =
=
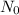
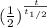
substituting values, we have
3.9 = 75 x
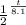
0.052 =
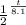
take the log of both side
log 0.052 = log
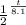
log 0.052 =
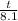 log 1/2
log 1/2
-1.284 =
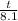 x -0.301
x -0.301
1.284 = 0.301t/8.1 =
1.284 = 0.0372t
t = 1.284/0.037 = 34.5 ≅ 35 hrs