Answer:
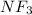
Step-by-step explanation:
Geometrical symmetry of the molecule and the polarity of the bonds determine the polarity of the molecule.
The molecule that has zero dipole moment that means it is a geometrically symmetric molecule and the molecule which has some net dipole moment means it is a geometrically asymmetric molecule.
As the molecule is symmetric, the dipole moment will be zero as dipole moments cancel each other and the molecule will be non-polar.
As the molecule is asymmetric, the dipole moment will not be zero and the molecule will be polar.
Example:
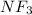
Thus, we can say that
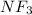 is a polar molecule.
is a polar molecule.