Answer:
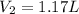
Step-by-step explanation:
Hello,
In this case, for dilution processes, we must remember that the amount of solute remains the same, therefore, we can write:
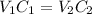
Whereas V accounts for volume and C for concentration that in this case is %(m/v). In such a way, the final volume V2 turns out:
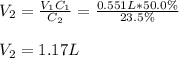
Best regards.