Answer:
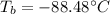
Step-by-step explanation:
Hello,
In this case, since the entropy of vaporization is defined in terms of the enthalpy of vaporization and the boiling point of the given substance, nitrous oxide, as shown below:
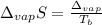
Solving for the boiling point of nitrous oxide, we obtain:
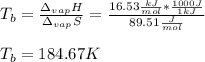
Which in degree Celsius is also:
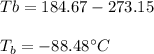
Best regards.