Answer:
The specific heat of the metal is 0.0482 joules per gram-Celsius.
Step-by-step explanation:
Block of metal is cooled by adding water and thermal equilibrium is reached. According to the First Law of Thermodynamics and by supposing the absence of energy and mass interaction of the system with surroundings, the change in the energy of the system is represented by:

Where
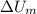 and
and
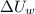 are the changes in internal energies of the block of metal and water, measured in joules.
are the changes in internal energies of the block of metal and water, measured in joules.
The expression described above is now extended by applying the definition of internal energy for constant mass systems:
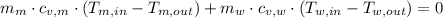
Where:
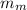 ,
,
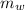 - Masses of the block of metal and water, measured in grams.
- Masses of the block of metal and water, measured in grams.
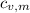 ,
,
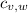 - Specific heats of the block of metal and water, measured in joules per gram-Celsius.
- Specific heats of the block of metal and water, measured in joules per gram-Celsius.
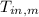 ,
,
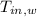 - Initial temperatures of the block of metal and water, measured in Celsius.
- Initial temperatures of the block of metal and water, measured in Celsius.
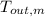 ,
,
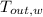 - Final temperatures of the block of metal and water, measured in Celsius.
- Final temperatures of the block of metal and water, measured in Celsius.
The specific heat of the metal is cleared in the equation:
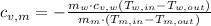
If
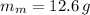 ,
,
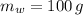 ,
,
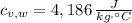 ,
,
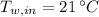 ,
,
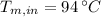 and
and
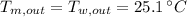 , the specific heat of the metal is:
, the specific heat of the metal is:
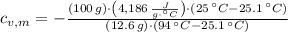
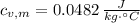
The specific heat of the metal is 0.0482 joules per gram-Celsius.