Answer:
The volume that a mass of 47.2 g would occupy is 0.0334 L
Step-by-step explanation:
Density is the property that matter, whether solid, liquid or gas, has to compress into a given space. Density is defined as the amount of mass it has per unit volume, that is, the ratio between the mass of a body and the volume it occupies:
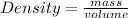
This indicates that density is inversely proportional to volume: the smaller the volume occupied by a given mass, the higher the density.
In this case:
- density= 1.413 g/mL
- mass= 47.2 g
- volume=?
Replacing:
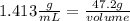
Solving:
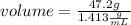
volume=33.40 mL
Being 1,000 mL= 1 L:
volume= 0.0334 L
The volume that a mass of 47.2 g would occupy is 0.0334 L