Answer:
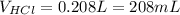
Step-by-step explanation:
Hello,
In this case, since the chemical reaction is:
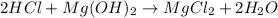
We can see that hydrochloric acid and magnesium hydroxide are in a 2:1 mole ratio, which means that the neutralization point, we can write:

In such a way, the moles of magnesium hydroxide (molar mass 58.3 g/mol) in 500 mg are:
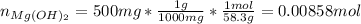
Next, since the pH of hydrochloric acid is 1.25, the concentration of H⁺ as well as the acid (strong acid) is:
![[H^+]=[HCl]=10^(-pH)=10^(-1.25)=0.0562M](https://img.qammunity.org/2021/formulas/chemistry/college/b4ekyij3k1gnc0w2y73fgw5tnrperfd26d.png)
Then, since the concentration and the volume define the moles, we can write:
![[HCl]*V_(HCl)=2*n_(Mg(OH)_2)](https://img.qammunity.org/2021/formulas/chemistry/college/fzf162cugtidh0yu8vcurwgql16tfj30q8.png)
Therefore, the neutralized volume turns out:
Best regards.