Answer:
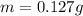
Step-by-step explanation:
Hello,
In this case, for a first-order reaction, we can firstly compute the rate constant from the given half-life:
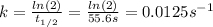
In such a way, the integrated first-order law, allows us to compute the final mass of the substance once 10.0 minutes (600 seconds) have passed:
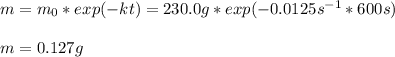
Best regards.