Answer:
222.3 ml of a 0.130 M aqueous solution of chromium (II) nitrate must be taken to obtain 5.08 grams of the salt.
Step-by-step explanation:
Being:
- Cr: 52 g/mole
- N: 14 g/mole
- O: 16 g/mole
the molar mass of chromium (II) nitrate, Cr(NO₃)₂ is:
Cr(NO₃)₂ = 52 g/mole + 2* (14 g/mole + 3* 16 g/mole)= 176 g/mole
So: if 176 grams are present in 1 mole of the compound, 5.08 grams in how many moles of the compound will be present?
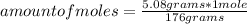
amount of moles=0.0289 moles
Molarity (M) is the number of moles of solute that are dissolved in a given volume. It is then calculated by dividing the moles of the solute by the volume of the solution:
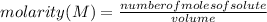
Molarity is expressed in

So in this case:
- molarity= 0.130 M
- number of moles of solute= 0.0289 moles
- volume= ?
Replacing:
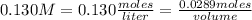
Solving:
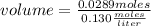
volume=0.2223 liters
Being 1 L= 1,000 mL:
volume=0.222 liters= 222.3 mL
222.3 ml of a 0.130 M aqueous solution of chromium (II) nitrate must be taken to obtain 5.08 grams of the salt.