Answer:
The value is

Step-by-step explanation:
From the question we are told that
The pressure is
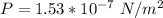
The temperature is
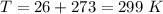
The volume is 1 cubic cm =
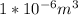
Generally according to the ideal gas law we have that
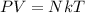
here k is the Boltzmann constant with a value
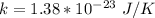
=>
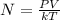
=>
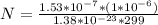
=>
