Answer:
The number of molecules is 4.574 x 10¹¹ Molecules
Step-by-step explanation:
Given;
pressure in the vacuum system, P = 1 x 10⁻⁹ Pa
volume of the vessel, V = 1.9 m³
temperature of the system, T = 28°C = 301 K
Apply ideal gas law;
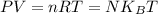
Where;
n is the number of gas moles
R is ideal gas constant = 8.314 J / mol.K
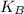 is Boltzmann's constant, = 1.38 x 10⁻²³ J/K
is Boltzmann's constant, = 1.38 x 10⁻²³ J/K
N is number of gas molecules
N = (PV) / (
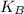 T)
T)
N = (1 x 10⁻⁹ X 1.9) / ( 1.38 x 10⁻²³ X 301)
N = 4.574 x 10¹¹ Molecules
Therefore, the number of molecules is 4.574 x 10¹¹ Molecules