Answer:
A : At the end of the hour, isotope B has a greater decay constant λ than isotope A
Step-by-step explanation:
Firstly, we need to understand that radioactive decay follows a first order rate law.
What this means is that we can calculate the radioactive decay constant using the following formula from the half-life
Mathematically;
 = 0.693/λ
= 0.693/λ
where λ represents the radioactive decay constant.
Rearranging the equation, we can have
λ = 0.693/

Now, to have a fair level playing ground, it is best that the half-life of both isotopes are in the same unit of time(seconds)
For A, the half-life = 2.3 minutes which is same as 2.3 × 60 = 138 seconds
For B, the half-life is 24 seconds
Thus, at the end of the hour, the decay constant for isotope A will be;
λ = 0.693/138 = 0.0050
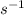
For isotope B, the decay constant will be;
λ = 0.693/24 = 0.028875
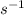
We can see that the decay constant of isotope B is higher than that of A at the end of the experiment