Answer:
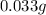
Step-by-step explanation:
Hello,
In this case, since 11 mg per kilogram of body weight has the given lethality, the mg that turn out lethal for a chicken weighting 3 kg is computed by using a rule of three:
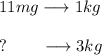
Thus, we obtain:
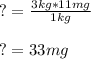
That in grams is:
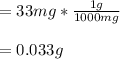
Regards.