Answer:
The rate at which ZnCl2 appears increases.
Step-by-step explanation:
Hello,
In this case, the reaction is:
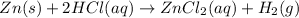
Therefore, the law of rate proportions is:
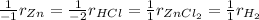
In such a way, since the concentration of hydrochloric acid is increasing The rate at which ZnCl2 appears increases, because the addition of a reactant is directly related with the products formation due to the fact that more reactant will yield more product.
Best regards.