Answer:
ethyl 4-bromobenzoate
Step-by-step explanation:
In this question, we can start with the Index of Hydrogen Deficiency (I.H.D):
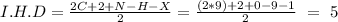
This indicates, that we can have a benzene ring (I.H.D = 4) and a carbonyl group (I.H.D = 1), for a total of 5.
Additionally, in the 1H-NMR info, we have a triplet 1.39 (3H) followed by a doublet 4.38 (2H), this indicates the presence of an ethyl group (
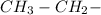 ). Also, in the formula, we have 2 oxygens if we have carbonyl group with 2 oxygens we have a high probability to have an ester group.
). Also, in the formula, we have 2 oxygens if we have carbonyl group with 2 oxygens we have a high probability to have an ester group.
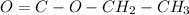
Now, if we add this to the benzene ring and the "Br" atom that we have in the formula, we will have ethyl 4-bromobenzoate.
See figures 1 and 2 to further explanations.
I hope it helps!