Answer:
B. 1-amino-2-methylpentan-2-ol
Step-by-step explanation:
In this case, the first step, we have the attack of the nucleophile cyanide (
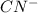 produced by sodium cyanide to the carbon on the carbonyl group (C=O) producing a negative charge in the oxygen.
produced by sodium cyanide to the carbon on the carbonyl group (C=O) producing a negative charge in the oxygen.
Then HCl protonates the molecule to produce a cyanohydrin. This cyanohydrin can be reduced by the action of hydrogen gas (
 ) in the presence of a catalyst (
) in the presence of a catalyst (
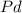 ), producing an amino group. With this in mind, the final molecule is: 1-amino-2-methylpentan-2-ol.
), producing an amino group. With this in mind, the final molecule is: 1-amino-2-methylpentan-2-ol.
See figure 1 to further explanations
I hope it helps!