Answer:
A. number of neutrons of Magnesium Mg = 13
B. The average mass of Mg = 22.29 amu
C. the magnesium composition on Mars is not the same as that on Earth.
Step-by-step explanation:
Isotopes are atoms with the same atomic number but different mass number. This is due to the difference in mass of the neutrons.
The atomic number of Magnesium Mg = 12
The atomic number of an element is the number of protons present in the atomic nucleus of the element
i.e Atomic number = number of protons = 12
The mass number of an element is the sum of the protons and neutrons in the atomic nucleus of the element.
Mass number = number of protons + number of neutrons
Given that the mass number of Mg = 25
Then;
25 = 12 + number of neutrons
25 - 12 = number of neutrons
13 = number of neutrons
number of neutrons of Magnesium Mg = 13
B. What is the average atomic mass of magnesium in these rocks?
The average atomic mass of an element which exhibit isotopy is the average mass of its various isotopes as they occur naturally in any quantity of the element.
Therefore the average atomic mass of magnesium can be calculated as:
=
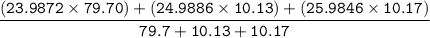
=
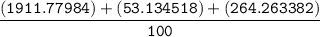
=
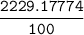
The average mass of Mg = 22.29 amu
C. Is the magnesium composition on Mars the same as that on Earth? Explain.
The average atomic weight of magnesium on Earth is said to be 24.305 amu while that of Mars is 22.29 amu.
There difference in the average atomic weight result into difference in their composition. Therefore,the magnesium composition on Mars is not the same as that on Earth.