Answer:
y = 1
Explanation:
The equation of a line is:
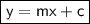
Here,
- m = slope of the line
- c = y-intercept
- y and x are variables
Finding the slope:
The slope of the line is zero, as the line is parallel to the x-axis.
Another way of finding the slope is by calculating the change in y concerning the change in x.
If we take two points that lie on the given line, like:
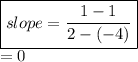
So, either way, we get the slope as zero.
=> m = 0
Finding the y-intercept:
The y-intercept of a line is the value of the y coordinate at which the line meets the y-axis, I.e.,
The point that lies on the line with its abscissa(x coordinate) zero.
Here, one such point is:
its y-intercept is 1.
=> c = 1
Finding the equation:
y = mx + c
y = 0 + 1
=> y = 1
That's the equation we've been looking for!