Answer:
d) NH4F
Step-by-step explanation:
Hello,
In this case, the base resulting from mixing a weak acid and a weak base is d) NH4F since ammonium hydroxide is a wear base and hydrofluoric acid is a weak acid.
Ammonium hydroxide is a weak base since it is not completely ionized in ammonium and hydroxyl ions:
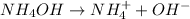
Moreover, hydrofluoric acid is a weak acid since it is not completely ionized in hydrogen and fluoride ions:
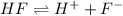
For the both of the substances, the limit is established by the basic and the acid dissociation constant respectively.
Regards.