Answer:
B) The complex ion is favored over solid silver chloride
C) The free Ag+ ion is unstable.
Step-by-step explanation:
Hello,
In this case, since the dissociation of solid silver chloride occurs at equilibrium with a neglectable solubility product (very small Ksp), which means that the solid tends to remain undissolved:
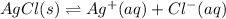
By the addition of ammonia, the following reaction is favored:
![Ag^+(aq)+2NH_3(aq)\rightleftharpoons [Ag(NH_3)_2]^+(aq)](https://img.qammunity.org/2021/formulas/chemistry/college/w809jrrtootdivp6wqv2dn0x9x85rl8usf.png)
Which has a large equilibrium constant, which means that the formation of the complex is assured. In such a way, by addition of more ammonia, more complex will be formed, therefore B) The complex ion is favored over solid silver chloride is true. Moreover, C) The free Ag+ ion is unstable, since they tend to form the complex once they are formed by the solid silver chloride so it readily reacts.
Best regards.