Answer:
d. 8 moles of H2O on the product side
Step-by-step explanation:
Hello,
In this case, we need to balance the given redox reaction in acidic media as shown below:
![MnO_4^(1-) (aq) + Cl^(1-) (aq) \rightarrow Mn^(2+) (aq) + Cl_2 (g)\\\\(Mn^(7+)O^(2-)_4)^(1-) (aq) + Cl^(1-) (aq) \rightarrow Mn^(2+) (aq) + Cl_2 (g)\\\\\\\\(Mn^(7+)O^(2-)_4)^(1-) (aq)+8H^++5e^- \rightarrow Mn^(2+)+4H_2O\\\\2Cl^(1-)\rightarrow Cl_2^0+2e^-\\\\2*[(Mn^(7+)O^(2-)_4)^(1-) (aq)+8H^++5e^- \rightarrow Mn^(2+)+4H_2O]\\\\5*[2Cl^(1-)\rightarrow Cl_2^0+2e^-]\\\\\\\\2(Mn^(7+)O^(2-)_4)^(1-) (aq)+16H^++10e^- \rightarrow 2Mn^(2+)+8H_2O\\\\10Cl^(1-)\rightarrow 5Cl_2^0+10e^-\\](https://img.qammunity.org/2021/formulas/chemistry/college/15coi1vtqpew1jlbogdxk1856ehh6d67uv.png)
Then, we add the half reactions:
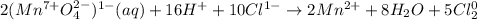
Thereby, we can see d. 8 moles of H2O on the product side.
Best regards.