Answer:
AgCl (silver Chloride) is being precipitated out as white and cloudy crystals.
Step-by-step explanation:
If a student mixes 1.0 mL of aqueous silver nitrate AgNO3 (aq) with 1.0 mL of aqueous sodium chloride, NaCl (aq), in a clean test tube.
The sodium chloride is being acidified with dilute trioxonitrate (V) acid. Then a few drops of silver trioxonitrate(V) is added afterwards. A white precipitate of silver chloride, which dissolves readily in aqueous ammonia indicates the presence of sodium chloride.
The reaction proceeds as follows:
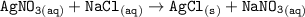
From the reaction between AgNO3 (aq) and NaCl (aq), AgCl (silver Chloride) is being precipitated out as white and cloudy crystals.