Answer:
1. 0.97 V
2.
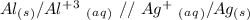
Step-by-step explanation:
In this case, we can start with the half-reactions:
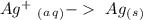
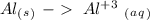
With this in mind we can add the electrons:
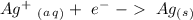 Reduction
Reduction
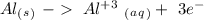 Oxidation
Oxidation
The reduction potential values for each half-reaction are:
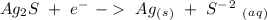 - 0.69 V
- 0.69 V
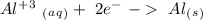 -1.66 V
-1.66 V
In the aluminum half-reaction, we have an oxidation reaction, therefore we have to flip the reduction potential value:
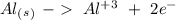 +1.66 V
+1.66 V
Finally, to calculate the overall potential we have to add the two values:
1.66 V - 0.69 V = 0.97 V
For the second question, we have to keep in mind that in the cell notation we put the anode (the oxidation half-reaction) in the left and the cathode (the reduction half-reaction) in the right. Additionally, we have to use "//" for the salt bridge, therefore:
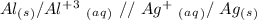
I hope it helps!