Answer:
Pentan-2-ol
Step-by-step explanation:
On this reaction, we have a Grignard reagent (ethylmagnesium bromide), therefore we will have the production of a carbanion (step 1). Then this carbanion can attack the least substituted carbon in the epoxide in this case carbon 1 (step 2). In this step, the epoxide is open and a negative charge is generated in the oxygen. The next step, is the treatment with aqueous acid, when we add acid the hydronium ion (
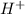 ) would be produced, so in the reaction mechanism, we can put the hydronium ion. This ion would be attacked by the negative charge produced in the second step to produce the final molecule: "Pentan-2-ol".
) would be produced, so in the reaction mechanism, we can put the hydronium ion. This ion would be attacked by the negative charge produced in the second step to produce the final molecule: "Pentan-2-ol".
See figure 1
I hope it helps!