Answer:
B.3/5p
Step-by-step explanation:
For this question, we have to remember "Dalton's Law of Partial Pressures". This law says that the pressure of the mixture would be equal to the sum of the partial pressure of each gas.
Additionally, we have a proportional relationship between moles and pressure. In other words, more moles indicate more pressure and vice-versa.
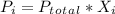
Where:
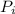 =Partial pressure
=Partial pressure
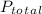 =Total pressure
=Total pressure
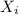 =mole fraction
=mole fraction
With this in mind, we can work with the moles of each compound if we want to analyze the pressure. With the molar mass of each compound we can calculate the moles:
moles of hydrogen gas
The molar mass of hydrogen gas (
 ) is 2 g/mol, so:
) is 2 g/mol, so:
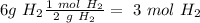
moles of oxygen gas
The molar mass of oxygen gas (
 ) is 32 g/mol, so:
) is 32 g/mol, so:
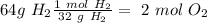
Now, total moles are:
Total moles = 2 + 3 = 5
With this value, we can write the partial pressure expression for each gas:
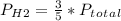
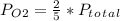
So, the answer would be 3/5P.
I hope it helps!